bromine valence electrons|Bromine Valence Electrons : Clark Bromine is extracted by electrolysis from natural bromine-rich brine deposits in the USA, Israel and China. It was the first element to be extracted from seawater, but this is now .
Browse 78 mods for Mad Max at Nexus Mods. In the wake of E3’s demise, Gamescom has settled in nicely as the king of gaming events, showing off the latest releases and - more excitedly - what’s to come!Speaking of what’s to come, I love speculating on the future.
PH0 · What Are Valence Electrons? Definition and Periodic Table
PH1 · Valence Electrons Chart for All Elements
PH2 · How to Find the Valence Electrons for Bromine (Br)?
PH3 · How to Find the Valence Electrons for Bromine (Br)
PH4 · How many valence electrons does bromine have?
PH5 · How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bro
PH6 · How Many Valence Electrons Does Bromine (Br) Have?
PH7 · Determine valence electrons using the periodic table
PH8 · Bromine Valence Electrons
PH9 · Bromine
PinayFlix - Watch all the best collection of movies and clips from PinayFlix
bromine valence electrons*******There are two ways to find the number of valence electrons in Bromine (Br). The first is to use the Periodic Table to figure out how many electrons Bromine h. Mar 23, 2023
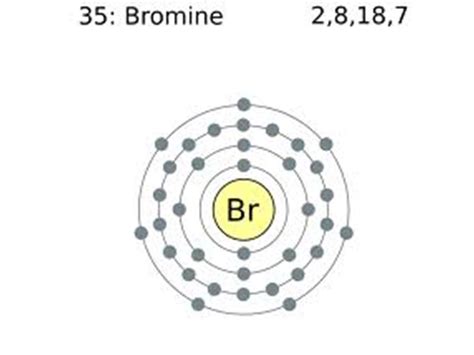
Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. The valence electrons are seven and the valency is one, based on the electron configuration .Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is .
Learn how to determine the number of valence electrons for an element using the periodic table. Bromine is in group 17 and has seven valence electrons. See examples, .Bromine is extracted by electrolysis from natural bromine-rich brine deposits in the USA, Israel and China. It was the first element to be extracted from seawater, but this is now . Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [Ar]4s 2 3d 6 . Iron is a transition metal, so the number of valence electrons .
Bromine Valence Electrons Dot Diagram. You can study the valence electrons of Bromine with the help of the Lewis dot diagram. The diagram breaks down the total numbers of Bromine valence electrons .Bromine is a chemical element; . chlorine, and iodine. Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all .
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
Bromine Valence Electrons The elements that receive electrons and form bonds are called anions. During the formation of a bond, the last shell of bromine receives an electron and turns into a bromide ion(Br –). That is, .
Explanation: Bromine is in family VII A. the same as Fluorine Chlorine. All members of the family have seven valance electron hence the name 7A. 7 only the electrons in the outmost shell are valance electrons.All but seven of the electrons in bromine are in lower shells Bromine is in family VII A. the same as Fluorine Chlorine.
You can study the valence electrons of Bromine with the help of the Lewis dot diagram. The diagram breaks down the total numbers of Bromine valence electrons of atoms. Dot diagram further helps in understanding the interaction of valence electrons. It draws the numbers of dots around the symbol of Br as the valence electrons.
Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s . Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd . Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable . As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871.The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons. In general, atoms are most stable, least reactive, when their outermost electron shell .In the periodic table, bromine is a group VIIA element with seven electrons in its last shell. Therefore, the total number of valence electrons = 7(2) = 14. 2. Total electron pairs exist in the form of lone pairs and bonds. Total electron pairs are calculated by dividing the total valence electron count by two.Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons . Method 2: From the Electron Configuration. If you want to find the valence electrons of bromine from its electron configuration, then you should know its electron configuration first. Now there are many methods to write the electron configurations, but here I will show you the easiest method, i.e by using Aufbau principle. Aufbau principle: .
The atomic number of Bromine Br is 35. The electronic configuration of Bromine Br can be written as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5; The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a total of seven valence electrons. Therefore, the valence .Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .

A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and .
bromine valence electrons A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and .
Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom. The electron configuration of a oxygen atom is. O: 1s22s22p4 (1.9B.1) (1.9B.1) O: 1 s 2 2 s 2 2 p 4. which may be shorted.
bromine valence electrons Bromine Valence Electrons There is only one element in bromine molecule. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of bromine atoms. valence electrons given by bromine atoms = 7 * 2 = 14; Total valence electrons = 14
BFP NCR Pasig City s n r S t e p o d o 8 c 8 t 4 2 r 2 1 a 2 0 9 o 2 3 2 i 0 h 5 1 1 a h N e v 8 1 e l 0 a f c 2 c 2 m 0 u , 1 9 b 3 2
bromine valence electrons|Bromine Valence Electrons